Development of new drugs:

Cardiotoxicity:
Secondary side-effects of a drug can lead to alterations in contractility, heart function and/or heart damage, and eventually to sudden death. This is one the most worrying secondary effects of drugs and the leading cause of drug withdrawal from market, both for drugs treating cardiovascular and non-heart related problems. Current withdrawals due to cardiotoxicity in human are still about 40%.
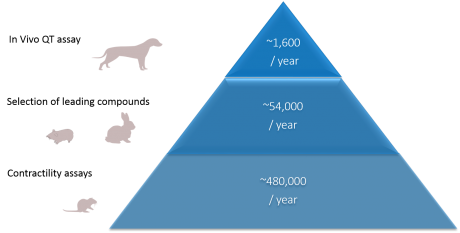
The use of animals:
Current strategies to study cardiotoxicity during the preclinical stage involve the use of a variety of animals (usually rats, mice, rabbits, guinea-pigs and dogs), exceeding more than 500,000 animals per year.
In spite of such a variety of animal methods, hundreds of thousands of these animals were not effective models of human cardiotoxicity as they were not able to detect liabilities in human. In addition, between 20% and 50% of advanced drug candidates have to be abandoned late in the drug development process due to adverse outcomes. An earlier identification of these adverse outcomes would represent a drastic reduction in the use of animal lives in industry, with an associated reduction in the economic cost of releasing new drugs to market.
In silico research:
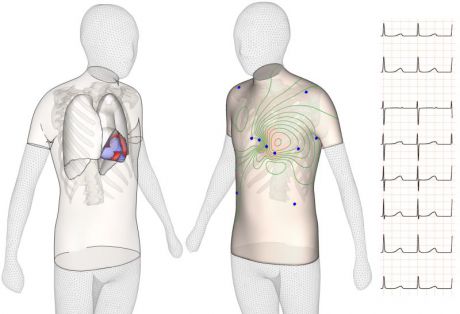
Over the last years, intensive research has been done in the development of in silico models that reproduce human electrophysiology from the cellular to the whole-organ (and body) levels.
Using a human-based in silico approach could yield reductions of up to 90% in animal usage. In silico methods are faster than animal research and can give predictive information of the outcomes of drugs on human. This would allow a human oriented pre-screening of compounds before testing on animals, refining and reducing animal usage by only testing those drugs considered safe in human. The use of human in silico models would bring additional advantages such as the reduction of patient risk during clinical trials, the reduction of failure rates in drug development, and speeding up the development of safer medicines to patients who are in urgent need of them.
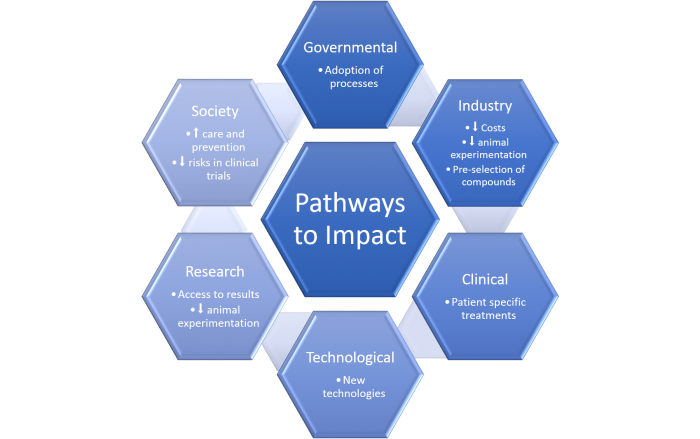
Our vision
Objectives:
The main aim of this consortium is to accelerate the uptake of human-based in silico methodologies for the evaluation of cardiac drug safety and efficacy in industrial, regulatory and clinical settings. We will work towards the refinement of human-based methodologies by compiling the existing knowledge on human and animal databases, human in silico models and expertise from all partners.
By bringing together academia, the pharmaceutical industry and regulatory agencies, this project creates the opportunity to overcome many of the time consuming and expensive challenges of cardiotoxicity research through the use of in silico methods.
Our objective is to demonstrate the power and address possible limitations of these models, to provide confidence for their approval by regulatory agencies and their uptake by institutions to firstly complement, and then gradually replace, refine and reduce the use of animals on drug research.

Impact:
By bringing together this multisectorial alliance of pharmaceutical, regulatory, clinical, technological and research stakeholders, our project aims to accelerate the intake of human-based computational methodologies for an effective reduction, refinement and replacement of animal experimentation in cardiac drug safety and efficacy.