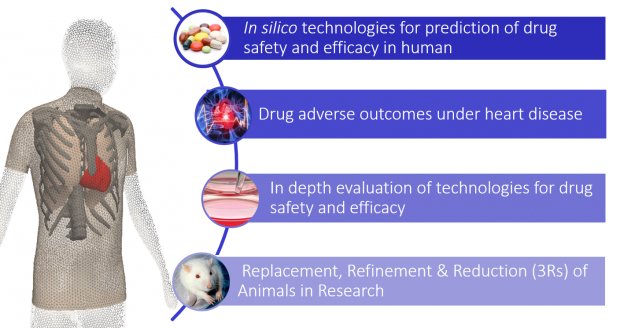
Cardiotoxicity (adverse outcomes of the heart) is a common side effect of many drugs, and the main reason for which a drug is withdrawn from the market. In addition to the high cost of releasing a new drug, the preclinical stage involves a large number of animals (more than 500,000 per year), raising both ethical and economic issues. Despite these high costs, the process is inefficient and more than 40% of new drugs are considered cardiotoxic when used in humans. This is in part due to clear differences between human and animal physiology, and the limited use of human-based selective and specific assays at preclinical stage.
Over the last decades, computational (in silico) models of human cardiac electrophysiology have been developed in health and disease, bridging from ionic to whole organ human physiology. Their translation to industry, clinical and regulatory settings requires multi-sectorial partnerships to evaluate the potential of the human in silico models in each setting, and facilitate their uptake.
The main goal of this initiative is to accelerate the use of in silico methodologies for the evaluation of drug safety and efficacy in academic, industrial, regulatory and clinical settings. We aim to increase the efficiency of drug testing through the use of human-based computer models refined and evaluated using experimental and clinical recordings. Through our research we will aim to the refinement, reduction and replacement of animals in the drug development process, and the development of a more reliable and accurate process for drug testing. Our team is formed by a partnership across industry, academia, hospitals and regulatory agencies from 14 different countries.
This webpage is an open space to share knowledge, papers, tools and models, and also a reference for experts on in silico methodologies for human drug safety and efficacy. We encourage all partners to get involved and send us news, papers, tools and models related to the project, to keep the website alive (Contact Us).
If you are interested in knowing more about the project and receiving our newsletter,
join our Mailing List!